量子化学分子モデリング(DFT/MM)に基づく検証-2
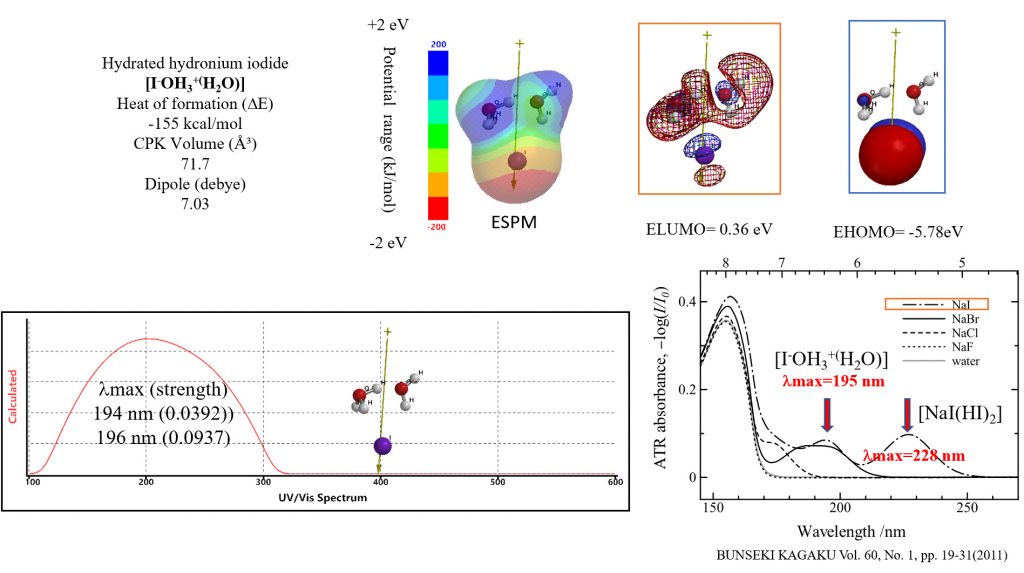
5. The iodide ion, which is a component of iodine omega 3, exists in the cell as hydrated hydroiodide [I–OH3+(H2O)].
Verification that the equilibrium three-dimensional structure of dilute iodide ions in cells is hydrated hydronium iodide [I–OH3+(H2O)]
The electrostatic potential map (ESPM) obtained by DFT/MM reflects the conformations of LUMO and HOMO, and verifies that van der Waals association with various molecules is easy. Especially, the negatively large electrostatic potential (red) on the iodine atom (attributable to HOMO configuration) predicts the existence of strong van der Waals bond to other molecules.
The UV/Vis spectrum measured in aueous dilute NaI solution is reported to show absorption maximum wavelengths, λmax=195nm nm and λmax=228 nm. In the diluted NaI solution, the dissociation of NaI into HI progressed, and we predicted that [NaI(HI)2] and [I–OH3+(H2O)] would coexist. The maximum wavelength of the UV/Vis spectrum based on each DFT/MM was given by lmax=195 nm and lmax=228 nm, and coincided with the observed absorption maximum wavelength of the UV/Vis spectrum. This verifies that the iodide ion in the cell has an equilibrium geometry of hydrated hydronium iodide [I–OH3+(H2O)].
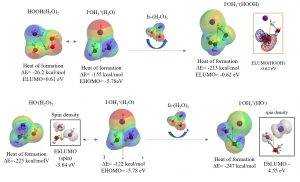
6. The hydroiodide [I–OH3+(H2O)] associates with [HOOH (H2O)2] to reduce its oxidizing power and suppress the generation of the bad active oxygen radical of [HO.(H2O)2] from HOOH. In addition, the hydroiodide [I–OH3+(H2O)] also associates with the bad hydroxyl radical [HO.(H2O)2] that was once generated, and loses its oxidative decomposition reactivity.
Verification of excellent antioxidant effect of iodide ion [I–OH3+(H2O)]
When incorporated into the body, iodide ions become hydrated hydroiodide [I–OH3+(H2O)] as mentioned. The iodide [I–OH3+(H2O))] reacts rapidly with hydrogen peroxide (HOOH) (DE= -213 kcal/mol) to become [I–OH3+(HOOH)] . As a result, the generation of hydroxyl radicals (HO.) from HOOH may be suppressed. Furthermore, even if hydroxyl radical (HO.) is generated, [I–OH3+(H2O)] reacts rapidly with HO. (ΔE= -247 kcal/mol) to give [I–OH3+HO.] . By localizing the radical site represented by the spin density to the iodide ion (I–], the hydrogen abstraction reaction of the radical is suppressed.
7. The radical [HO.I–OH3+], whose reactivity is inactivated by [I–OH3+] cannot destroy the cell membrane.
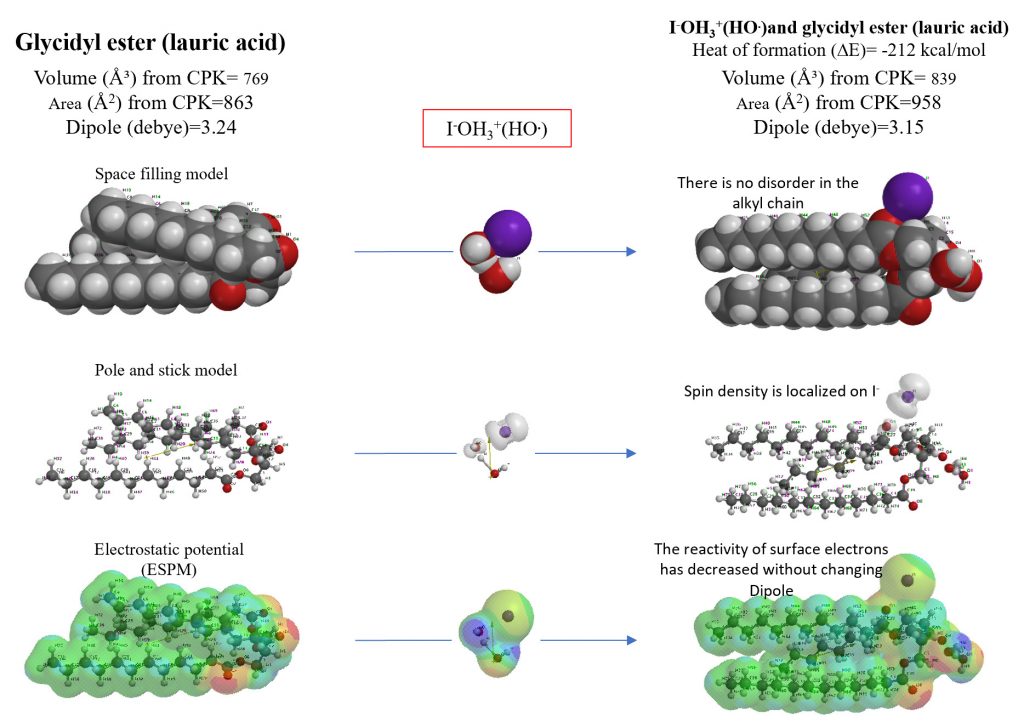
Verification of antioxidant effect of iodide ion (I–) on mitochondria
The reaction of hydroxyl radical of [I–OH3+(HO.)] with glycidyl ester of mitochondrial membrane composition molecule is shown by space filling (CPK) model, pole and stick model, and ESPM display. [I–OH3+(HO.)] reacts immediately with glycidyl ester but does not change the glycidyl ester structure significantly. This verifies and predicts the manifestation of the antioxidant effect due to the adsorption of iodide ion (I–) on the mt film.
8. The hydroiodide [I–OH3+(H2O)] is associated with omega-3 fatty acids, transported to mt, and adsorbed on the mt membrane. It serves as a preventative antioxidants to mt membrane.
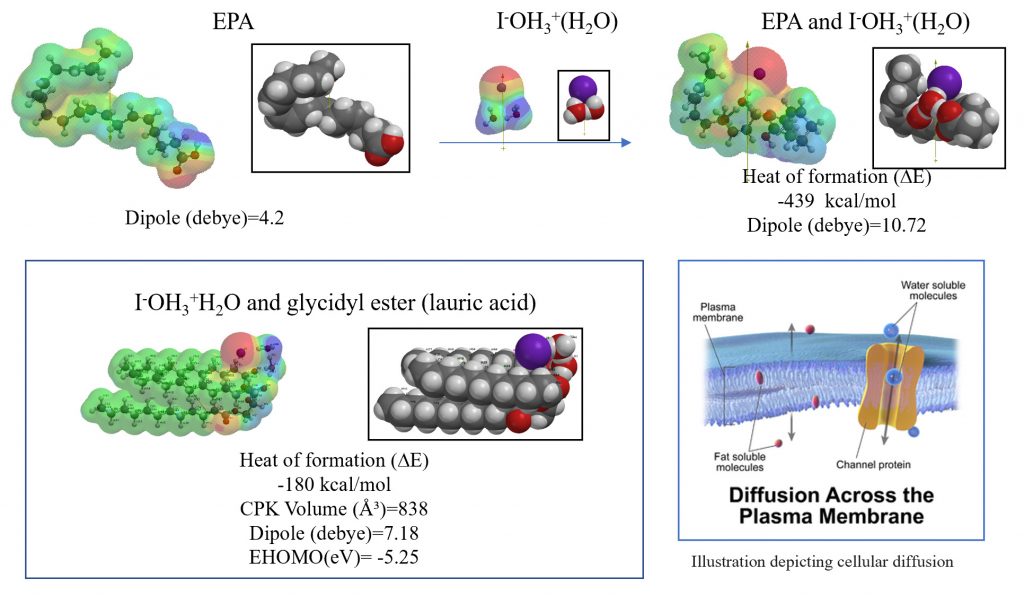
Verification of strong van der Waals binding between iodide ion [I–OH3+(H2O)] and omega-3 fatty acids
It was verified that the heat of formation (ΔE) of the association reaction of iodide ion [I–OH3+H2O] with EPA, which is an omega-3 fatty acid, is -439 kcal/mol, which is extremely large. Particularly noteworthy is that in the EPA aggregate of [EPA and I–OH3+(H2O)], the iodide ion associates with the double bond at the omega-3 position of EPA, resulting in a nearly spherical (or boat-like) EPA aggregate during the association process. Then, iodide ion [I–OH3+H2O and EPA] can flow smoothly in blood vessels by forming a spherical of boat-like structure with EPA. The iodide ion [I–OH3+H2O] that diffuses into the cells will be adsorbed to the glycidyl ester sites that compose the cell membrane and mt membrane. The heat of formation (ΔE) of the association reaction of [I–OH3+H2O] with the fatty acid glycidyl ester is exothermic at -180 kcal/mol, and the intracellular equilibrium adsorption of iodide ion is verified. Please refer to illustration depicting cellular diffusion with CPK volume of the iodide aggregates.
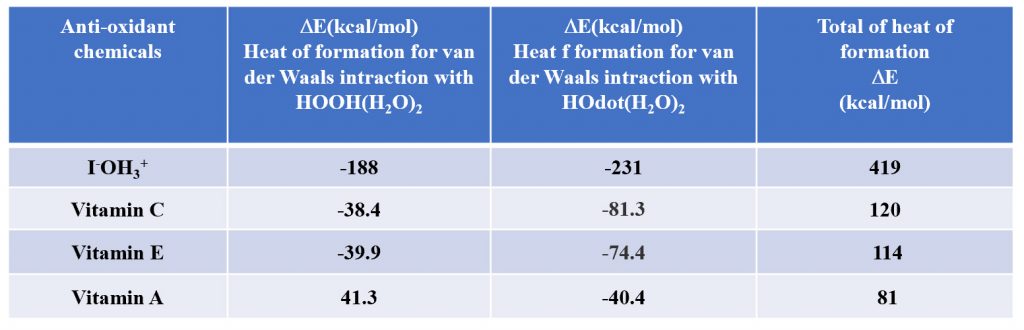
9. The heats of reaction between [HOOH (H2O)2] and [HO.(H2O)2] of hydroiodide [I–OH3+] were determined based on DFT/MM, and compared with the heats of reaction of vitamins C, A and E, respectively. Hydroiodide [I–OH3+] clearly has an antioxidant effect superior to vitamins C, A, and E.
Verification that the antioxidant power of iodide ion is superior to vitamins C, A and E
The heat of formation at the time of association of iodide ion (I–OH3+) with hydrogen peroxide [HOOH(H2O)2] and hydroxyl radical [HO.(H2O)2] are determined by DFT/ MM. Similar heats of formation were obtained for Vitamin C, E, and A, and the antioxidant power was evaluated based on the total numerical value as shown below. The order is iodide,>>Vitamin C> vitamin E> vitamin A.
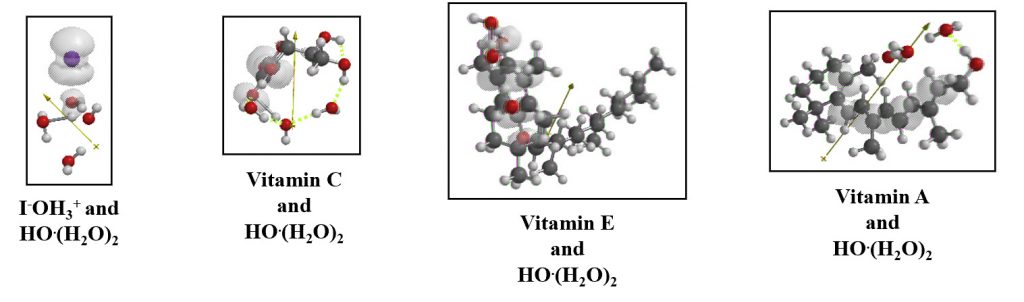
The figures below show the spin (radical) density distribution in the van der Waals aggregates of each antioxidant with [HO.(H2O)2]. In all cases, it is verified that the spin (radical) density is delocalized and the reaction force of hydrogen abstraction is suppressed.
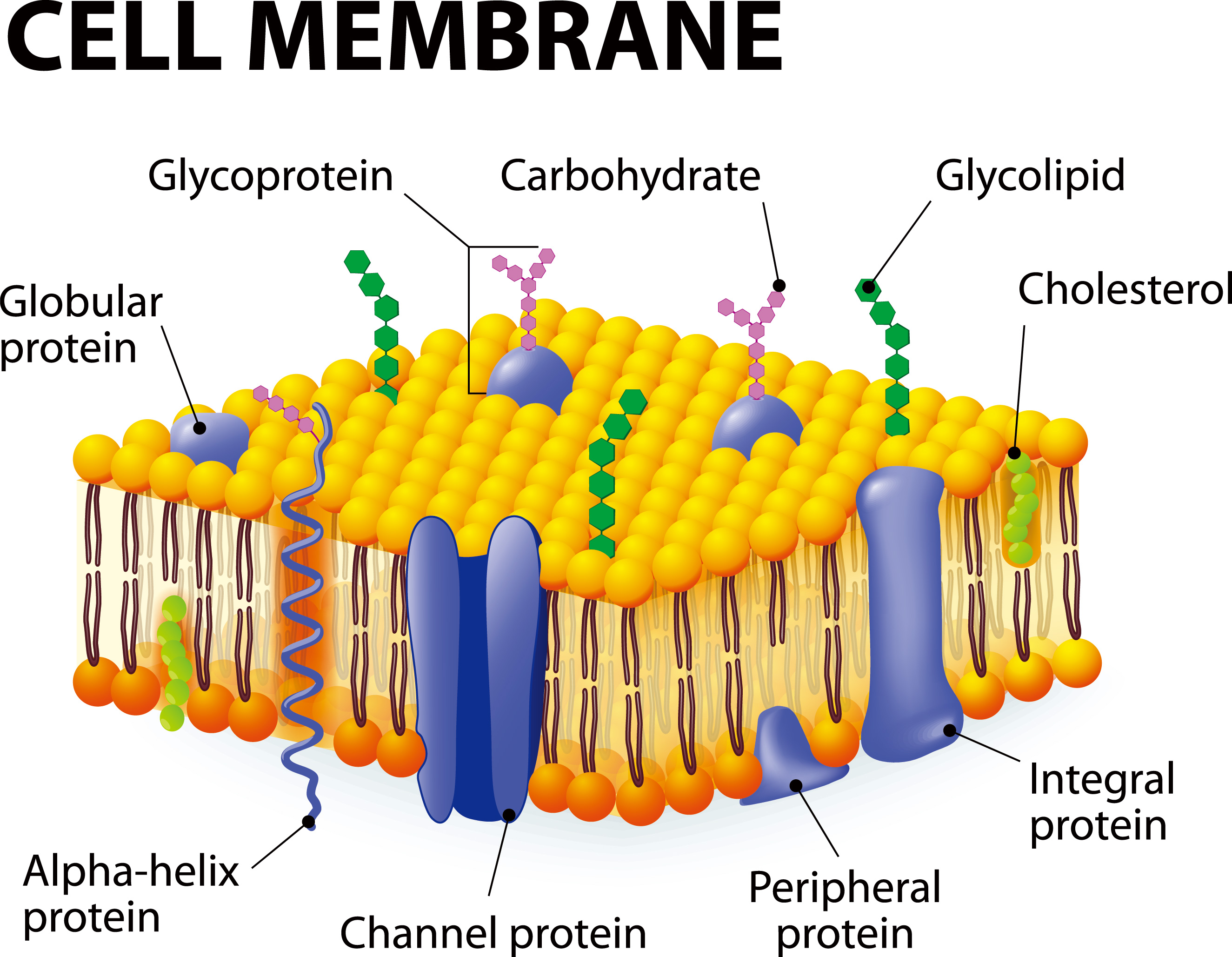
Accumulation of HOOH and formation of hydroxyl radical on mt lipid membrane lead to their structural disorder, which may be observed as swelling of mt and cells.
Mt dysfunction gradually starts as the disorder starts.